18 Types of High-risk Human Papilloma Virus Nucleic Acid
Product name
HWTS-CC018B-18 Types of High-risk Human Papilloma Virus Nucleic Acid Detection Kit (Fluorescence PCR)
Certificate
CE
Epidemiology
Cervical cancer is one of the most common malignant tumors in the female reproductive tract. Studies have shown that persistent infection and multiple infections of human papillomavirus are one of the important causes of cervical cancer.
Reproductive tract HPV infection is common among women with sexual life. According to statistics, 70% to 80% of women may have HPV infection for once at least in their lifetime, but most infections are self-limiting, and more than 90% of infected women will develop an effective immune response that could clear the infection between 6 and 24 months without any long-term health intervention. Persistent high-risk HPV infection is the main cause of cervical intraepithelial neoplasia and cervical cancer.
The worldwide study results showed that the presences of high-risk HPV DNA were detected in 99.7% of cervical cancer patients. Therefore, the early detection and prevention of cervical HPV are the key to blocking canceration. The establishment of a simple, specific and rapid pathogenic diagnostic method is of great significance in the clinical diagnosis of cervical cancer.
Channel
| FAM | HPV 18 |
| VIC (HEX) | HPV 16 |
| ROX | HPV 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82 |
| CY5 | Internal control |
Technical Parameters
| Storage | ≤-18℃ in dark |
| Shelf-life | 12 months |
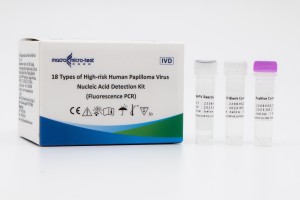
| Specimen Type | Cervical Swab、 Vaginal Swab 、Urine |
| Ct | ≤28 |
| CV | ≤5.0% |
| LoD | 300Copies/mL |
| Specificity | (1)Interfering Substances Use the kits to test the following interfering substances, the results are all negative: hemoglobin, white blood cells, cervical mucus, metronidazole, Jieryin lotion, Fuyanjie lotion, human lubricant.(2)Cross-reactivity Use the kits to test the other reproductive tract related pathogens and human genomic DNA that may have cross-reactivity with the kits, the results are all negative: HPV6 positive samples, HPV11 positive samples, HPV40 positive samples, HPV42 positive samples, HPV43 positive samples, HPV44 positive samples, HPV54 positive samples, HPV67 positive samples, HPV69 positive samples, HPV70 positive samples, HPV71 positive samples, HPV72 positive samples, HPV81 positive samples, HPV83 positive samples, herpes simplex virus type Ⅱ, treponema pallidum, ureaplasma urealyticum, mycoplasma hominis, candida albicans, neisseria gonorrhoeae, trichomonas vaginalis, chlamydia trachomatis and human genomic DNA |
| Applicable Instruments | SLAN-96P Real-Time PCR Systems
Applied Biosystems 7500 Real-Time PCR Systems Applied Biosystems 7500 Fast Real-Time PCR Systems QuantStudio®5 Real-Time PCR Systems LightCycler®480 Real-Time PCR Systems LineGene 9600 Plus Real-Time PCR Detection Systems MA-6000 Real-Time Quantitative Thermal Cycler BioRad CFX96 Real-Time PCR System BioRad CFX Opus 96 Real-Time PCR System |
Total PCR Solution
Option 1.
1. Sampling
2. Nucleic acid extraction

3. Add samples to the machine

Option 2.
1. Sampling

2. Extraction-free

3. Add samples to the machine