Malaria Nucleic Acid
Product name
HWTS-OT074-Plasmodium Nucleic Acid Detection Kit(Fluorescence PCR)
HWTS-OT054-Freeze-dried Plasmodium Nucleic Acid Detection Kit (Fluorescence PCR)
Certificate
CE
Epidemiology
Malaria (Mal for short) is caused by Plasmodium, which is a single-celled eukaryotic organism, including Plasmodium falciparum Welch, Plasmodium vivax Grassi & Feletti, Plasmodium malariae Laveran, and Plasmodium ovale Stephens. It is a mosquito-borne and blood-borne parasitic disease that seriously endangers human health.
Of the parasites that cause malaria in humans, Plasmodium falciparum Welch is the deadliest. The incubation period of different malaria parasites is different, the shortest is 12-30 days, and the longer one can reach about 1 year. After the paroxysm of malaria, symptoms such as chills and fever may appear. The patients may have anemia and splenomegaly. The serious patients may have coma, severe anemia, acute renal failure which may lead to the death of patients. Malaria is distributed worldwide, mainly in tropical and subtropical regions such as Africa, Central America, and South America.
Channel
| FAM | Plasmodium nucleic acid |
| VIC (HEX) | Internal control |
Technical Parameters
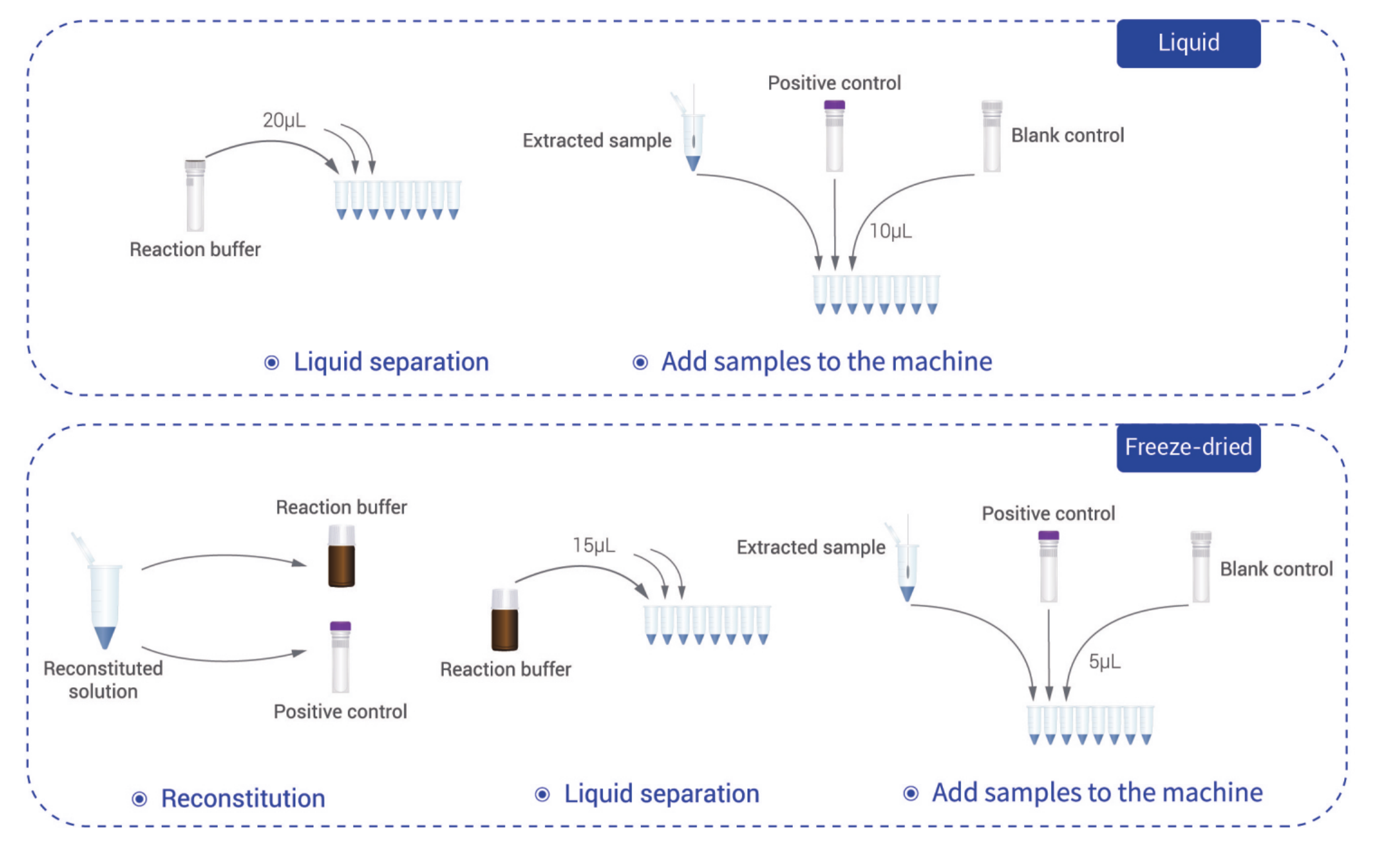
| Storage | Liquid: ≤-18℃ In dark; Lyophilized: ≤30℃ In dark |
| Shelf-life | 12 months |
| Specimen Type | Whole blood, dried blood spots |
| Ct | ≤38 |
| CV | ≤5.0% |
| LoD | 5Copies/μL |
| Repeatability | Detect the company repeatability reference and calculate the coefficient of variation CV of Plasmodium detection Ct and the result≤ 5% (n=10). |
| Specificity | No cross reactivity with influenza A H1N1 virus, H3N2 influenza virus, influenza B virus, dengue fever virus, encephalitis B virus, respiratory syncytial virus, meningococcus, parainfluenza virus, rhinovirus, toxic bacillary dysentery, staphylococcus aureus, escherichia coli, streptococcus pneumoniae or klebsiella pneumoniae, salmonella typhi, and rickettsia tsutsugamushi, and the test results are all negative. |
| Applicable Instruments | It can match the mainstream fluorescent PCR instruments on the market.
SLAN-96P Real-Time PCR Systems |